News
Vaccines used in Mauritius ‘do not meet EU criteria’
Hours after the first shipment of 60,000 doses of Russia’s Sputnik V COVID-19 vaccine was delivered to Mauritius on Wednesday, the European Union’s much anticipated coronavirus digital certificate, which is intended to facilitate unrestricted movement across the continent for vaccinated travelers, will begin Thursday amid widespread confusion about how the rollout will go.
But many vaccinated would-be travelers — including from Mauritius — do not meet the program’s criteria because they received AstraZeneca shots produced by India’s Serum Institute, which has not been approved by E.U. regulators.
So far, only four Western-produced vaccines qualify under the EU certificate’s criteria: Moderna, Pfizer-BioNTech, Johnson & Johnson and AstraZeneca doses, also called Vaxzevria, manufactured in Europe by AstraZeneca, which developed the vaccine with Oxford University.
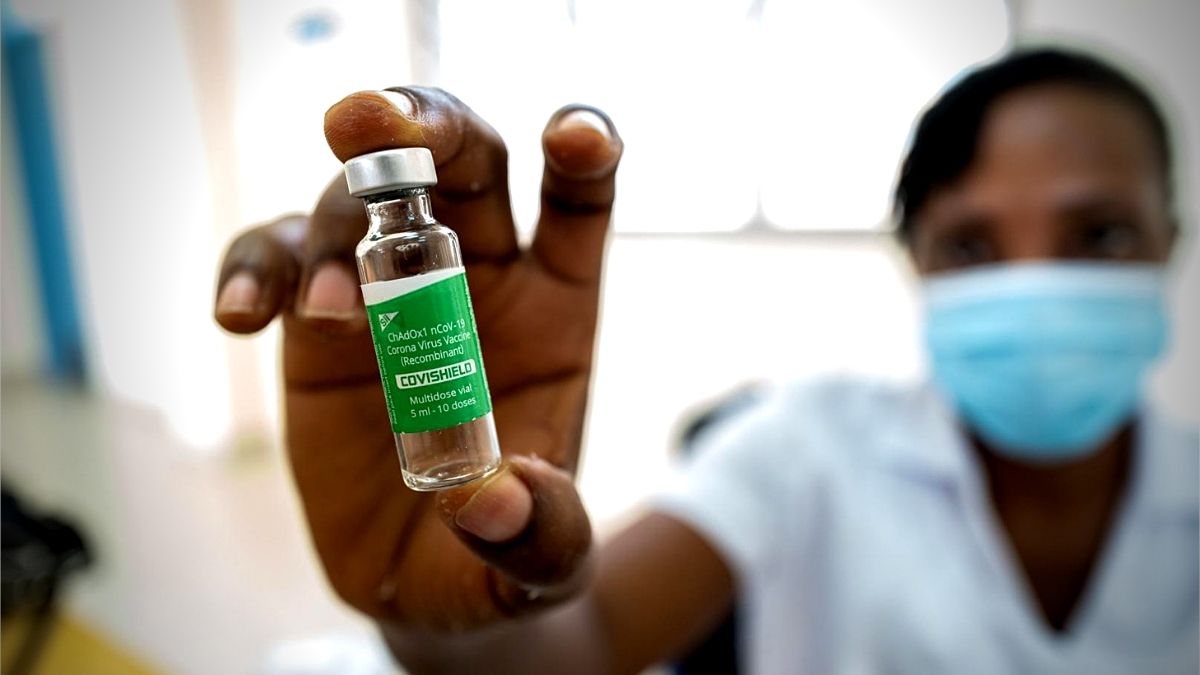
Not on the list is the Serum Institute’s AstraZeneca vaccine version, called Covishield, as it is not approved by the European Medicines Agency, the European Union’s medical regulatory body.
Also not included are the Russian Sputnik V and the Chinese Sinovac.
These are the only three vaccines available and used in Mauritius.
Under the rules, which are not obligatory for EU member states to follow, each country can also choose to waive quarantines for travelers vaccinated against the coronavirus with versions approved by the WHO.











